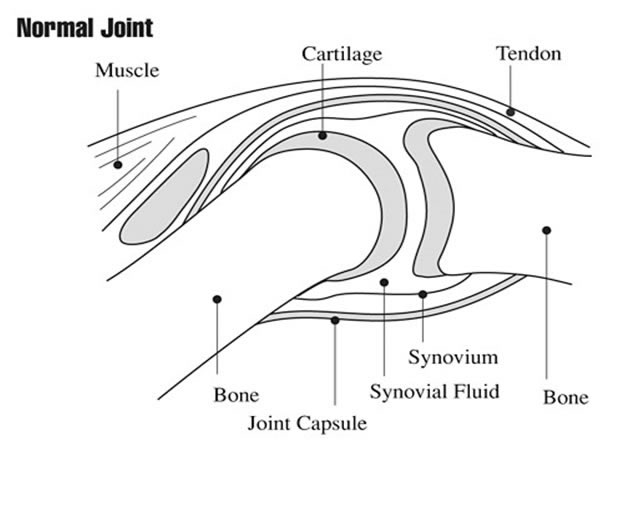
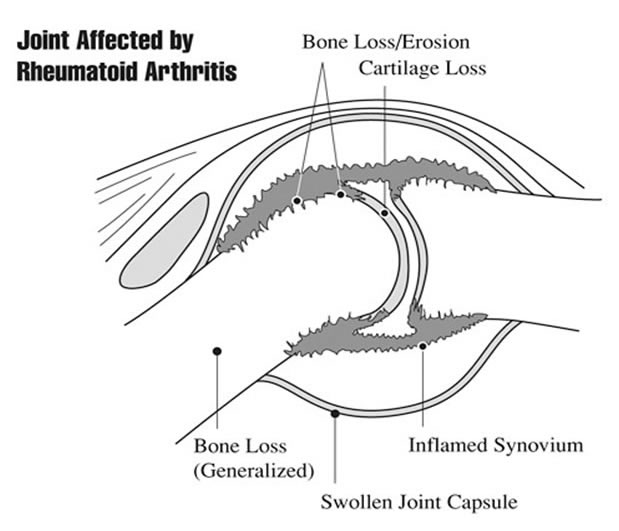
A normal healthy joint is lined by the so-called synovial membrane, formed by synoviocytes, also known as fibroblast-like synoviocytes (FLS), stromal cells that produce the fluid that lubricates and feed the joint surface. In patients with inflammatory arthritis, the synovial membrane is infiltrated by cells of the innate and adaptive immunity, which leads to the proliferation of FLS, and the thickening of the intimal layer, accompanied by the growth of new blood vessels. Although these are well-described general mechanisms that drive the inflammation of the synovial membrane, today we know that the same mechanisms are not active in all patients and in all diseases at the same time. The PEAC study aimed at studying the synovial membrane in patients with early inflammatory arthritis, i.e. before they started any treatment, and has contributed to identify a great deal of heterogeneity between individual diseases (e.g. Rheumatoid Arthritis or Psoriatic Arthritis) and within individual patients with the same disease, as described below, taking Rheumatoid arthritis as an example.